Strahle lab

STRAHLE LAB
Washington University in Saint Louis
STRAHLE LAB vision
Our goal is to understand germinal matrix hemorrhage-intraventricular hemorrhage (GMH-IVH)-induced brain injury and hydrocephalus in order to develop targeted therapeutics to prevent hydrocephalus, mitigate brain injury, and improve outcomes in infants with GMH-IVH. We study the role of blood and blood breakdown products in the pathogenesis of hydrocephalus and brain injury and examine the role of cilia on the ventricular surface in this pathogenesis. We also focus on the changes in CSF circulation and fluid dynamics after hemorrhage and CSF-neuronal interactions in development. Our multidisciplinary approach spans molecular, cellular, genetic, imaging, and behavioral techniques to answer basic science and clinical questions. We are integrated into the Hope Center for neurologic disorders at Washington University in Saint Louis and are located in BJC-Institute of Health, which provides for a strong interdisciplinary research program.
Selected PUBLICATIONS

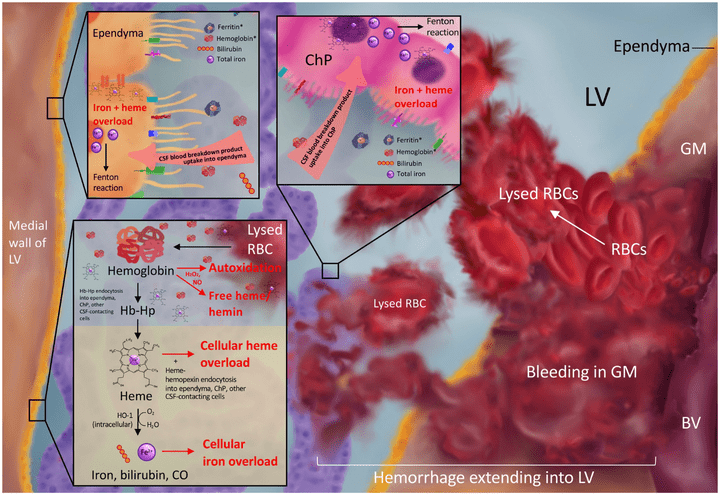
Iron homeostasis and post-hemorrhagic hydrocephalus: a review
Front. Neurol. 2024 Jan 12; Volume 14- 2023.
Iron physiology is regulated by a complex interplay of extracellular transport systems, coordinated transcriptional responses, and iron efflux mechanisms. Dysregulation of iron metabolism can result in defects in myelination, neurotransmitter synthesis, and neuronal maturation. In neonates, germinal matrix-intraventricular hemorrhage (GMH-IVH) causes iron overload as a result of blood breakdown in the ventricles and brain parenchyma which can lead to post-hemorrhagic hydrocephalus (PHH). However, the precise mechanisms by which GMH-IVH results in PHH remain elusive. Understanding the molecular determinants of iron homeostasis in the developing brain may lead to improved therapies. This manuscript reviews the various roles iron has in brain development, characterizes our understanding of iron transport in the developing brain, and describes potential mechanisms by which iron overload may cause PHH and brain injury. We also review novel preclinical treatments for IVH that specifically target iron. Understanding iron handling within the brain and central nervous system may provide a basis for preventative, targeted treatments for iron-mediated pathogenesis of GMH-IVH and PHH.
Access full-length article here: https://www.frontiersin.org/articles/10.3389/fneur.2023.1287559/full

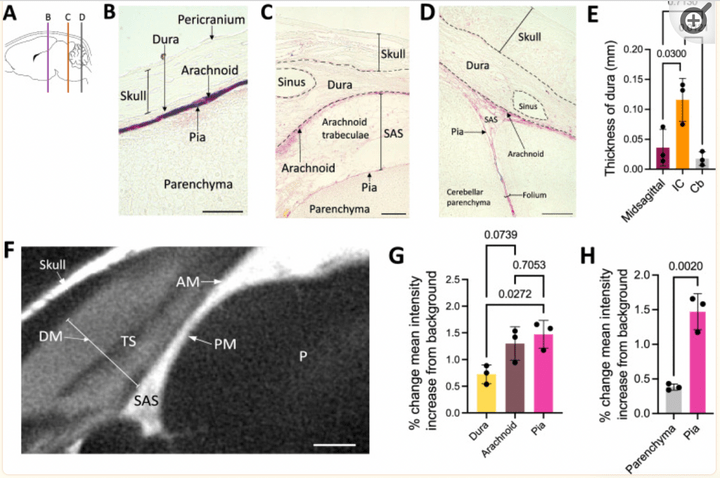
Postnatal meningeal CSF transport is primarily mediated by the arachnoid and pia maters and is not altered after intraventricular hemorrhage-posthemorrhagic hydrocephalus.
Fluids Barriers CNS. 2024 Jan 8;21(1):4.
There was significantly less CSF tracer distribution in the dura compared to the arachnoid and pia maters in neonatal rodents. Both small and large CSF tracers were transported intracranially to the arachnoid and pia mater of the perimesencephalic cisterns and tela choroidea, but not the falx cerebri. CSF tracers followed a similar distribution pattern in the spinal meninges. In the choroid plexus, there was large CSF tracer distribution in the apical surface of epithelial cells, and small CSF tracer along the basolateral surface. There were no significant differences in tracer intensity in the intracranial meninges of control vs. intraventricular hemorrhage-posthemorrhagic hydrocephalus (PHH) rodents, indicating preserved meningeal transport in the setting of PHH.
Access full-length article here: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10773070/

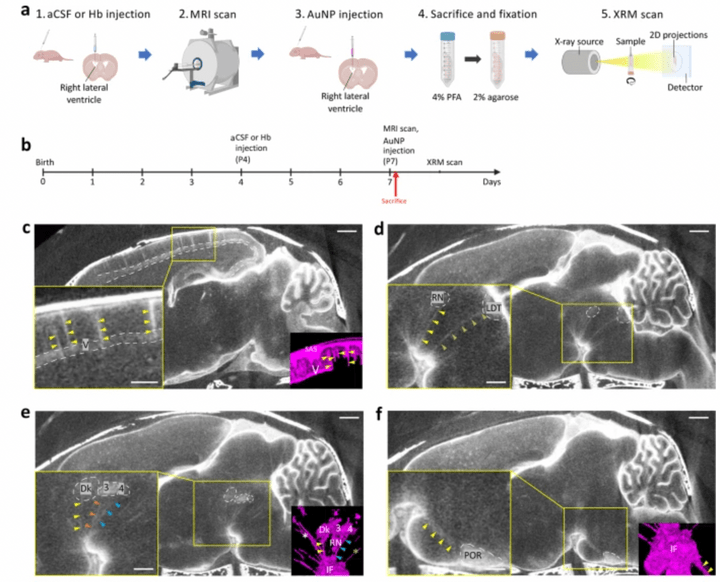
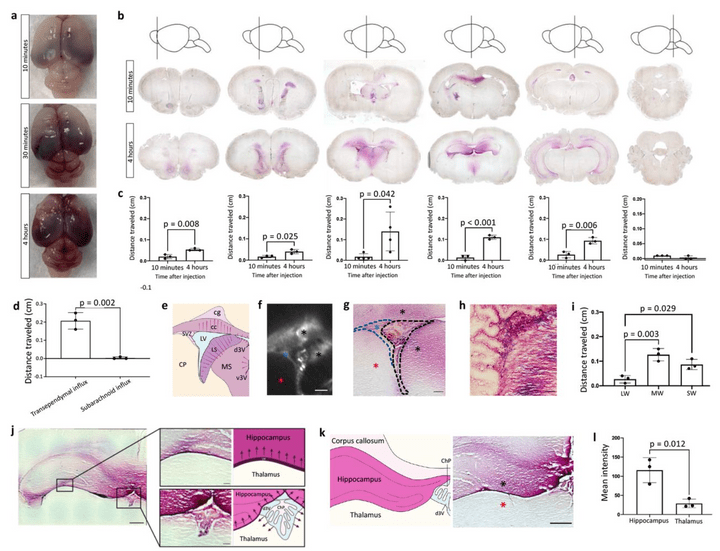
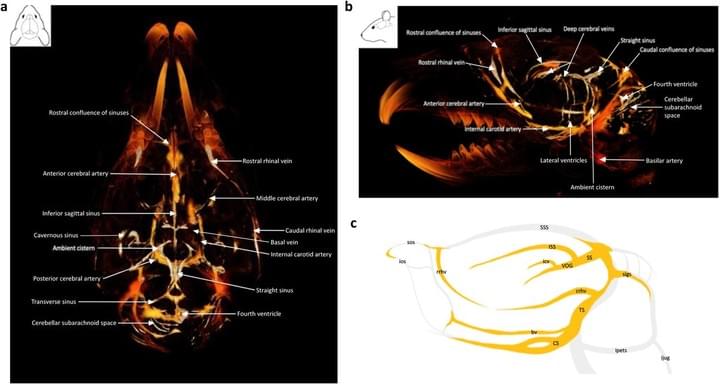
Gold nanoparticle-enhanced X-ray microtomography of the rodent reveals region-specific cerebrospinal fluid circulation in the brain
We develop a technique for CSF tracking, gold nanoparticle-enhanced X-ray microtomography, to achieve micrometer-scale resolution visualization of CSF circulation patterns during development. Using this method and subsequent histological analysis in rodents, we identify previously uncharacterized CSF pathways from the subarachnoid space (particularly the basal cisterns) that mediate CSF-parenchymal interactions involving 24 functional-anatomic cell groupings in the brain and spinal cord.
Access full-length article here: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9883388/

Deferoxamine Prevents Neonatal Posthemorrhagic Hydrocephalus Through Choroid Plexus-Mediated Iron Clearance
Transl Stroke Res. 2022 Oct. 10.1007/s12975-022-01092-7
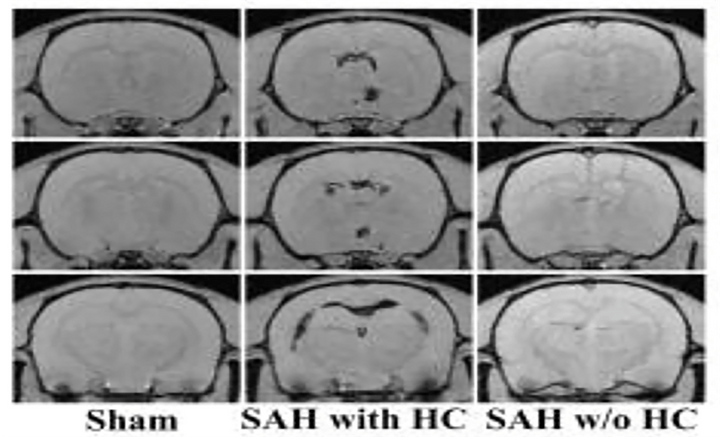
Hydrocephalus is an important complication of subarachnoid hemorrhage (SAH). SAH was induced by endovascular perforation in adult male Sprague-Dawley rats. Ventricular volume measurements showed a significant increase in surviving SAH rats when compared to sham controls. Ventricular volume correlated with SAH severity as demonstrated by histology, immunohistochemistry, Perls’ staining and Western blot analysis. Periventricular iron deposition was observed and HO-1 and Iba-1 expression were markedly increased in hydrocephalus rats.
Access full-length article here: https://pubmed.ncbi.nlm.nih.gov/36308676/

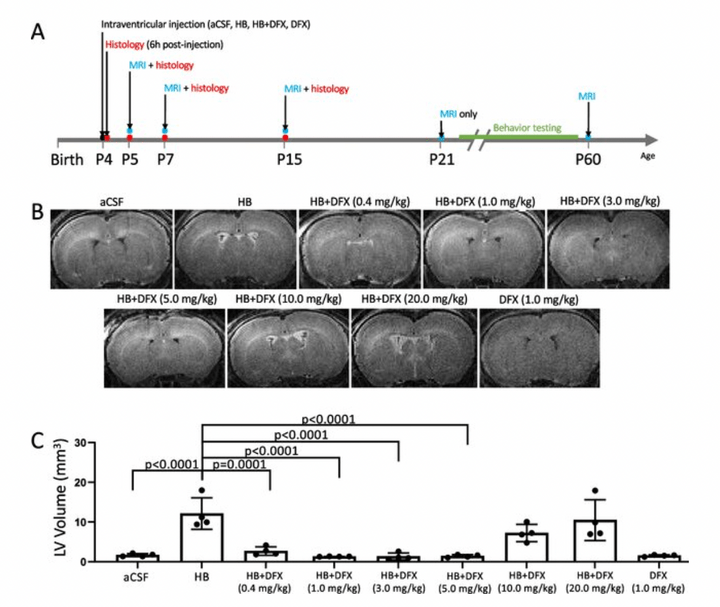
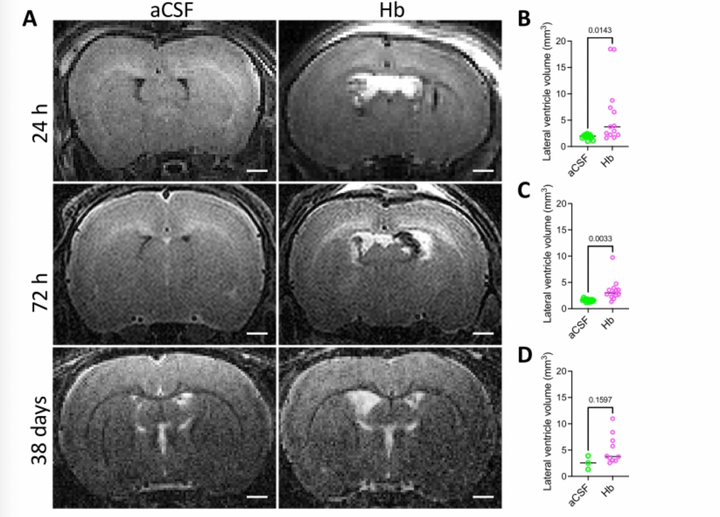
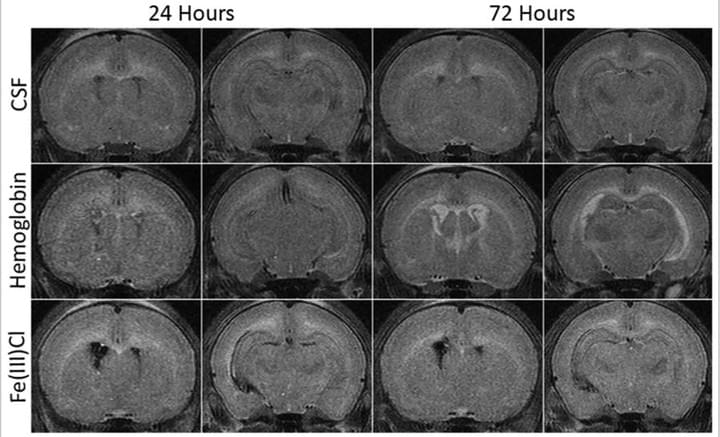
Modeling Neonatal Intraventricular Hemorrhage through Intraventricular Injection of Hemoglobin
JoVE. 2022 Aug 25;10.3791/63345.
We develop a straightforward animal model that mimics the clinical phenotype of PHH utilizing small-volume intraventricular injections of the blood breakdown product hemoglobin. In addition to reliably inducing ventricular enlargement and hydrocephalus, this model results in white matter injury, inflammation, and immune cell infiltration in periventricular and white matter regions. This paper describes this clinically relevant, simple method for modeling IVH-PHH in neonatal rats using intraventricular injection and presents methods for quantifying ventricle size post injection.
Access full-length article here: https://www.jove.com/t/63345/modeling-neonatal-intraventricular-hemorrhage-through
Etiology- and region-specific characteristics of transependymal cerebrospinal fluid flow
J Neurosurg Pediatr. 2022 Aug 12;1-11.
Transependymal flow (TEF) of CSF, often delineated as T2-weighted hyperintensity adjacent to the lateral ventricles on MRI, is a known imaging finding, usually in the setting of CSF flow disturbances. Specific radiological features of TEF and their relationships with clinical markers of hydrocephalus and underlying disease pathology are not known. Here, the authors describe the radiological features and clinical associations of TEF with implications for CSF circulation in the setting of intracranial pathology.
Access full-length article here: https://pubmed.ncbi.nlm.nih.gov/35962970/

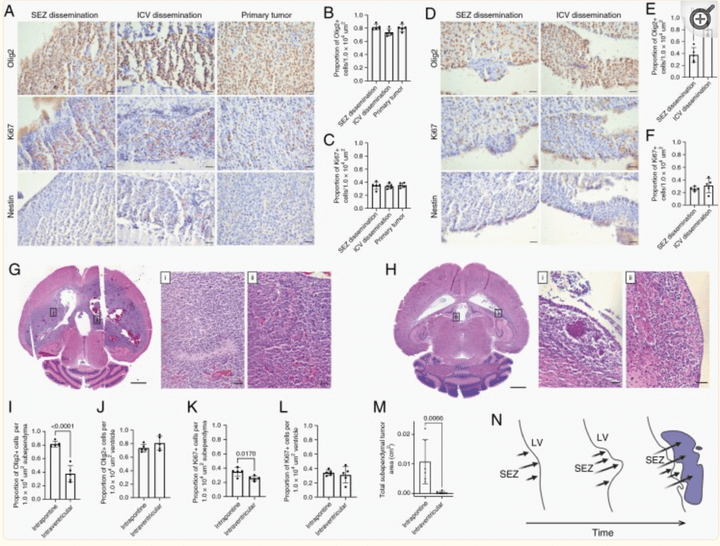
Leptomeningeal Disease and Tumor Dissemination in a Murine DIPG Model: Implications for Study of the Tumor-CSF-Ependymal Microenvironment
Neurooncol. Adv. 2022 Apr 26;4(1):vdac059.
This is the first study to report CSF pathway tumor dissemination associated with subependymal tumor in an animal model of DIPG and is representative of CSF dissemination seen clinically. Understanding the CSF-tumor-ependymal microenvironment has significant implications for treatment of DIPG through targeting mechanisms of tumor spread within the CSF pathways.
Access full-length article here: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9209751/

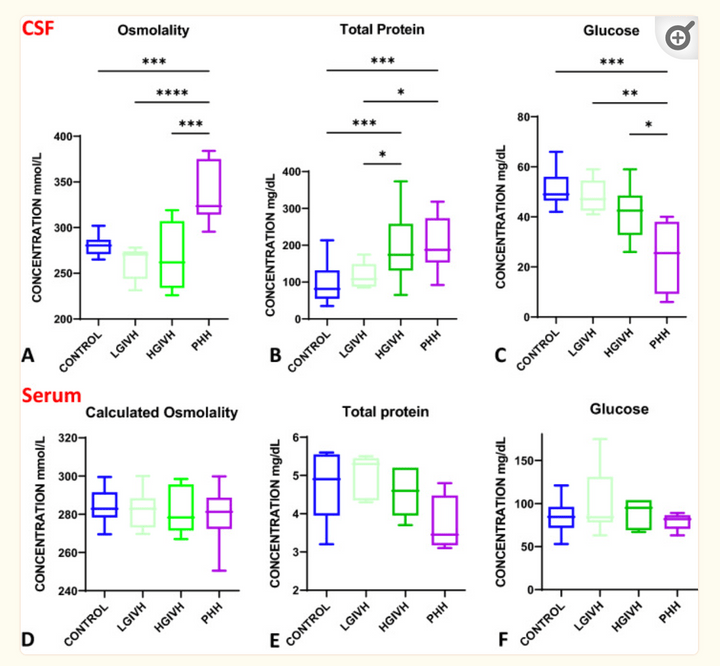
Biochemical profile of human infant cerebrospinal fluid in intraventricular hemorrhage and post-hemorrhagic hydrocephalus of prematurity
Fluids Barriers CNS. 2021 Dec 24;18(1):62.
Intraventricular hemorrhage (IVH) and post-hemorrhagic hydrocephalus (PHH) have a complex pathophysiology involving inflammatory response, ventricular zone and cell-cell junction disruption, and choroid-plexus (ChP) hypersecretion. CSF osmolality increased in PHH, largely driven by electrolyte changes rather than protein levels. However, serum electrolytes levels were unchanged across groups. CSF osmolality and electrolyte changes were correlated with CSF total nucleated cells which were also increased in PHH, further suggesting PHH is a neuro-inflammatory condition.
Access full-length manuscript here: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8710025/
Elevated cerebrospinal fluid iron and ferritin associated with early severe ventriculomegaly in preterm posthemorrhagic hydrocephalus
J Neurosurg Pediatr. 2022 May 27;30(2):169-176.
We evaluated CSF from serial ventricular taps to determine whether neonates with PHH following severe initial ventriculomegaly had higher initial levels and prolonged clearance of CSF hemoglobin and hemoglobin degradation products compared to those in neonates with PHH following moderate initial ventriculomegaly. Among preterm neonates with PHH following severe IVH, elevated CSF hemoglobin, ferritin, and iron were associated with more severe early ventricular enlargement (FOHR > 0.6 vs ≤ 0.6 at first ventricular tap).
Access full-length article here: https://pubmed.ncbi.nlm.nih.gov/35916101/
Genetic and histopathological associations with outcome in pediatric pilocytic astrocytoma
J Neurosurg Pediatr. 2022 Feb11;1-9.
The aim of this study was to identify the prognostic value of genetic and histopathological features of pediatric PAs. Histopathological features/qualifiers and BRAF alterations were not associated with tumor recurrence/progression in pediatric PAs. The extent of resection was the only factor analyzed that predicted outcome.
Access full-length article here: https://pubmed.ncbi.nlm.nih.gov/35148515/

Global cerebrospinal fluid circulation mapping using gold nanoparticle enhanced X-ray microtomography reveals region-specific brain and spinal cord CSF pathways
Preprint (bioRxiv)
We developed a novel technique for CSF tracking, gold nanoparticle enhanced X-ray microtomography, to achieve micrometer-scale resolution visualization of CSF pathways during development. Using this method and subsequent histological analysis, we map global CSF pathways and present novel particle size-dependent circulation patterns through the CNS. We identify an intraparenchymal CSF circulation that targets stem cell-rich and cholinergic neuronal populations. CSF solute distribution to these areas is mediated by CSF flow along projections from the basal cisterns which is altered in posthemorrhagic hydrocephalus.
Access full-length manuscript here: https://doi.org/10.1101/2021.12.18.473250
Children with supratentorial midline pilocytic astrocytomas exhibit multiple progressions and acquisition of neurologic deficits over time
Neurooncol Adv. 2021 Dec 27;4(1):vdab187.
To determine whether tumor location has any value in predicting PA clinical outcome, we evaluated clinical outcomes of children with biopsy-proven PA treated at St. Louis Children’s Hospital between 2003 and 2021 (n = 251).
Access full-length article here: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8757579/
Microstructural Periventricular White Matter Injury in Post-Hemorrhagic Ventricular Dilatation
Neurology. 2021 Nov 19;98(4):e364-e375.
The neurological deficits of neonatal post-hemorrhagic hydrocephalus (PHH) have been linked to periventricular white matter injury. To improve understanding of PHH-related injury, diffusion basis spectrum imaging (DBSI) was applied in neonates, modeling axonal and myelin integrity, fiber density, and extra-fiber pathologies. PHH was associated with diffuse white matter injury, including tract-specific patterns of axonal and myelin injury, fiber loss, cellular infiltration, and inflammation. Larger ventricular size was associated with greater disruption. Postmortem immunohistochemistry confirmed MRI findings. These results demonstrate DBSI provides an innovative approach extending beyond conventional diffusion MRI for investigating neuropathological effects of PHH on neonatal brain development.
Access full-length manuscript here: https://pubmed.ncbi.nlm.nih.gov/34799460/

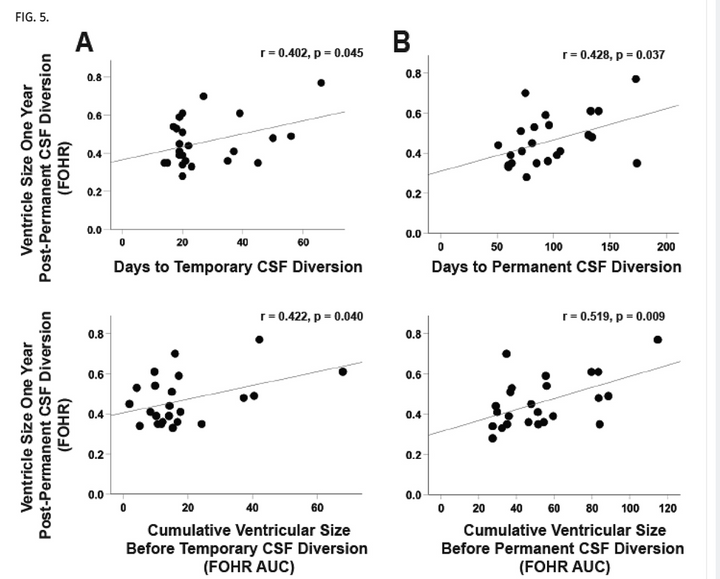
Does ventricle size contribute to cognitive outcomes in posthemorrhagic hydrocephalus? Role of early definitive intervention
J Neurosurg Pediatr. 2021 Oct 15;29(1):10-20.
Posthemorrhagic hydrocephalus (PHH) is associated with significant morbidity, smaller hippocampal volumes, and impaired neurodevelopment in preterm infants. The timing of temporary CSF (tCSF) diversion has been studied; however, the optimal time for permanent CSF (pCSF) diversion is unknown. The objective of this study was to determine whether cumulative ventricle size or timing of pCSF diversion is associated with neurodevelopmental outcome and hippocampal size in preterm infants with PHH.
Access full-length article here: https://pubmed.ncbi.nlm.nih.gov/34653990/
Longitudinal CSF Iron Pathway Proteins in Posthemorrhagic Hydrocephalus: Associations with Ventricle Size and Neurodevelopmental Outcomes
Ann Neurol. 2021 Aug;90(2):217-226.
Iron has been implicated in the pathogenesis of brain injury and hydrocephalus after preterm germinal matrix hemorrhage-intraventricular hemorrhage, however, it is unknown how external or endogenous intraventricular clearance of iron pathway proteins affect the outcome in this group. Longitudinal changes in CSF transferrin (increase) and ferritin (decrease) are associated with improved neurodevelopmental outcomes in neonatal PHH, with implications for understanding the pathogenesis of poor outcomes in PHH.
Access full-length article here: https://pubmed.ncbi.nlm.nih.gov/34080727/
Tract-Specific Relationships Between Cerebrospinal Fluid Biomarkers and Periventricular White Matter in Posthemorrhagic Hydrocephalus of Prematurity
Neurosurgery. 2021 Feb 16;88(3):698-706.
The objective of this study was to examine the relationship between diffusion magnetic resonance imaging (dMRI) and cerebrospinal fluid (CSF) biomarkers of PHH. Tract-specific associations were observed between dMRI and CSF biomarkers at the initiation of PHH treatment. dMRI and CSF biomarker analyses provide innovative complementary methods for examining PHH-related white matter injury and associated developmental sequelae.
Access full-length article here: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7884147/

Use of fast-sequence spine MRI in pediatric patients
J Neurosurg Pediatr. 2020 Sep 18;26(6):676-681.
The immediate and long-term risk of anesthesia in the pediatric population is controversial. Traditional spine MRI protocols require the patient to remain still during the examination, and in young children this frequently results in the need for sedation administration. The authors' goal was to develop an abbreviated spine MRI protocol to reduce sedation administration in young patients undergoing spine MRI. The authors report the first pediatric series of a fast spine MRI protocol for use in young patients. The protocol does not require sedation and is able to identify and monitor syrinx, spinal dysraphism, and potentially other intraspinal anomalies.
Access full-length article here: https://pubmed.ncbi.nlm.nih.gov/32947256/

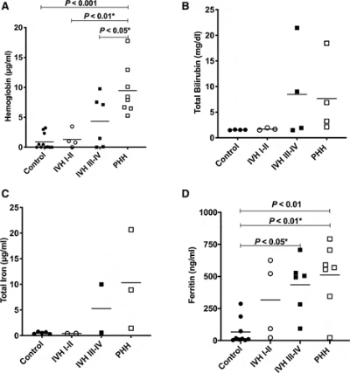
Intraventricular Hemorrhage Clearance in Human Neonatal Cerebrospinal Fluid: Associations With Hydrocephalus
2020 Jun;51(6):1712-1719.
Iron has been implicated in ventriculomegaly, hippocampal injury, and poor outcomes following IVH. The purpose of this paper to was to see whether levels of cerebrospinal fluid blood breakdown products and endogenous iron clearance proteins in neonates with IVH differ from those of neonates with IVH who subsequently develop posthemorrhagic hydrocephalus. Neonates with posthemorrhagic hydrocephalus had significantly higher levels of hemoglobin than those with high-grade IVH. Levels of blood breakdown products, hemoglobin, ferritin, and bilirubin correlated with ventricular size. There was no elevation of several iron-scavenging proteins in cerebrospinal fluid in neonates with posthemorrhagic hydrocepehalus, indicative of posthemorrhagic hydrocephalus as a disease state occurring when endogenous iron clearance mechanisms are overwhelmed.
Access full-length article here:
https://pubmed.ncbi.nlm.nih.gov/32397930/

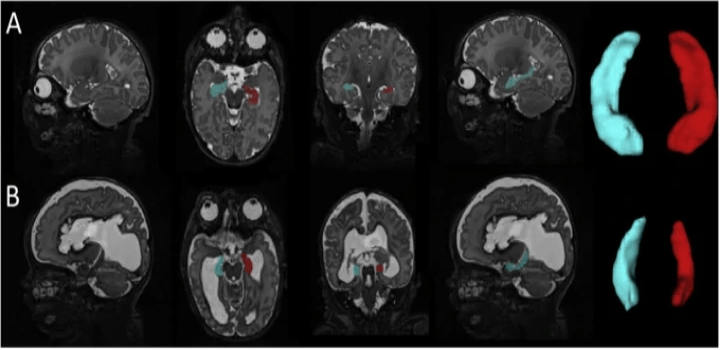
Impaired hippocampal development and outcomes in very preterm infants with perinatal brain injury
Neuroimage Clin. 2019; 22: 101787.
Preterm infants are at high risk for brain injury during the perinatal period. Intraventricular hemorrhage and periventricular leukomalacia, the two most common patterns of brain injury in prematurely-born children, are associated with poor neurodevelopmental outcomes. The hippocampus is known to be critical for learning and memory; however, it remains unknown how these forms of brain injury affect hippocampal growth and how the resulting alterations in hippocampal development relate to childhood outcomes. To investigate these relationships, hippocampal segmentations were performed on term equivalent MRI scans from 55 full-term infants, 85 very preterm infants (born ≤32 weeks gestation) with no to mild brain injury and 73 very preterm infants with brain injury (e.g., grade III/IV intraventricular hemorrhage, post-hemorrhagic hydrocephalus, cystic periventricular leukomalacia). Consistent with our preclinical findings, these findings demonstrate that perinatal brain injury is associated with hippocampal size in preterm infants, with smaller volumes related to domain-specific neurodevelopmental impairments in this high-risk clinical population.
Access full-length article here:

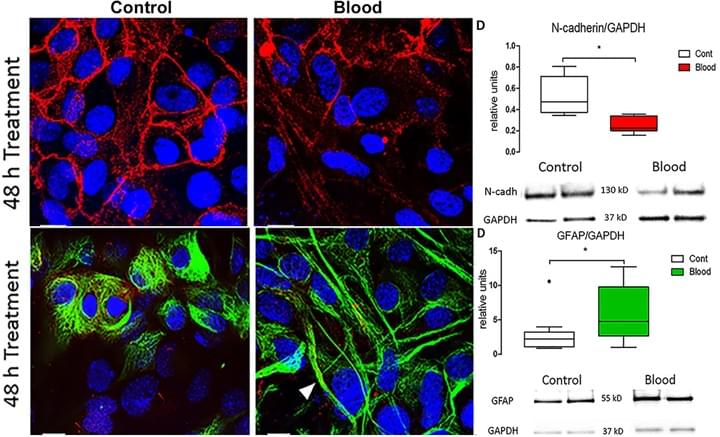
Blood Exposure Causes Ventricular Zone Disruption and Glial Activation In Vitro
J Neuropathol Exp Neurol. 2018 Sep 1;77(9):803-813
We reported that application of syngeneic blood to cultured ventricular zone (VZ) cells from newborn (P0-P4) mice resulted in significant decrease in N-cadherin-dependent adherenjunctions, ciliated ependymal cells and increase in glial fibrillary acidic protein. These observations suggest that, in vitro, blood triggers VZ cell loss and glial activation in a pattern that mirrors the cytopathology of human IVH and supports the relevance of this in vitro model to define injury mechanisms.
Access full-length article here: https://www.ncbi.nlm.nih.gov/pubmed/30032242

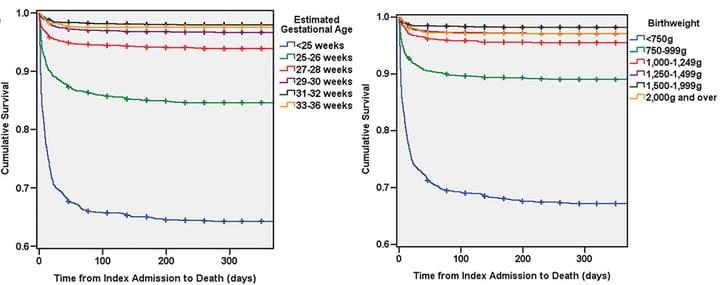
Predictors of mortality for preterm infants with intraventricular hemorrhage: a population-based study
Childs Nerv Syst. 2018 Jul 9
The goal of this longitudinal, population-level study was to examine factors affecting mortality in preterm infants with intraventricular hemorrhage (IVH). Patients were 36 weeks estimated gestational age (EGA) or less with a diagnosis of IVH. Potential predictors for mortality were investigated with multivariable survival analysis. Results are shown above. This is the first database for population-based investigations of long-term neurosurgical outcomes in preterm infants with IVH.
Access full-length article here: https://www.ncbi.nlm.nih.gov/pubmed/29987373

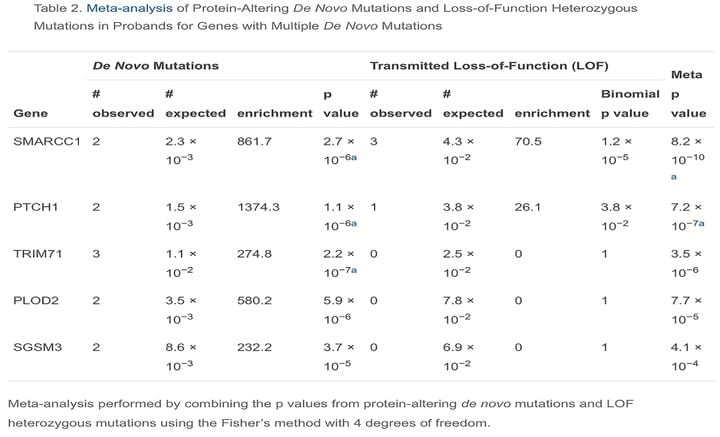
De Novo Mutation in Genes Regulating Neural Stem Cell Fate in Human Congenital Hydrocephalus
Neuron. 2018 Jul 25;99(2):302-314
CH pathogenesis is poorly understood. Exome sequencing of 125 CH trios and 52 additional probands identified three genes with significant burden of rare damaging de novo or transmitted mutations: TRIM71 (p = 2.15 × 10−7), SMARCC1 (p = 8.15 × 10−10), and PTCH1 (p = 1.06 × 10−6). Additionally, two denovoduplicationswere identified at the SHH locus, encoding the PTCH1 ligand (p = 1.2 × 10−4). Together, these probands account for ∼10% of studied cases. Strikingly, all four genes are required for neural tube development and regulate ventricular zone neural stem cell fate.
Access full-length article here: https://www.ncbi.nlm.nih.gov/pubmed/29983323

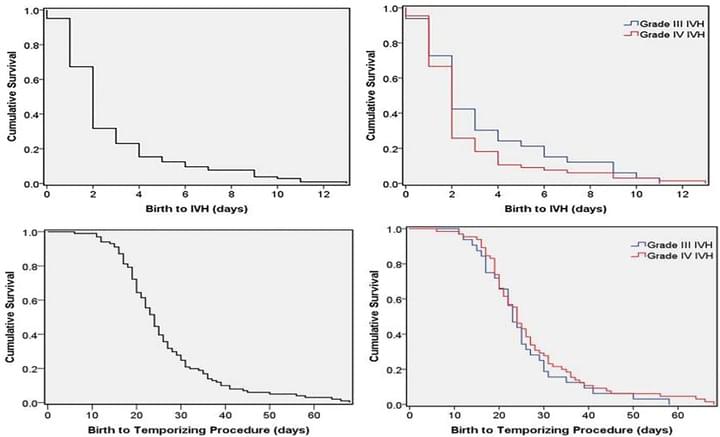
Time-to-event analysis of surgically treated posthemorrhagic hydrocephalus in preterm infants: a single-institution retrospective study
Childs Nerv Syst. 2017 Nov;33(11):1917-1926
Grades III-IV IVH did not differ in age at IVH diagnosis, ventriculomegaly, temporizing neurosurgical procedure (TNP) or permanent intervention. Ventricular reservoirs and ventriculosubgalealshunts were used in 68.3% and 28.8%, respectively. 76.9% of the patients ultimately received a VPS. Although most infants who develop IVH and ventriculomegaly will do so within a few days of birth, at-risk infants should be observed for at least four weeks with serial head ultrasounds to monitor for PHH requiring surgery.
Access full-length article here: https://www.ncbi.nlm.nih.gov/pubmed/28884229

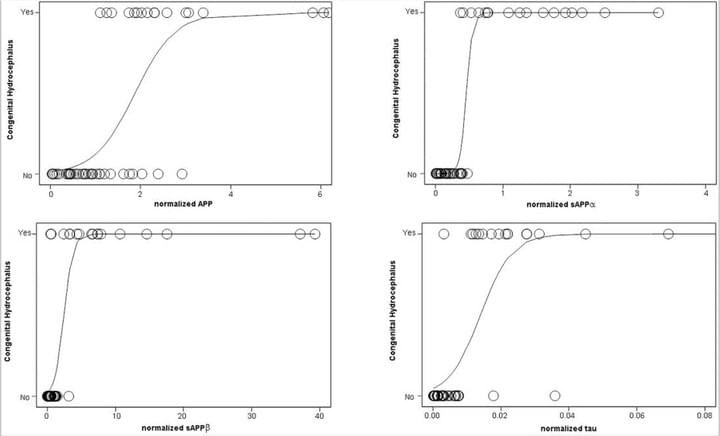
Cerebrospinal fluid biomarkers of infantile congenital hydrocephalus
PLoS One. 2017 Feb 17;12(2):e0172353
The objective of this study was to examine the CSF of children with congenital hydrocephalus (CHC) to gain insight into the pathophysiology of hydrocephalus and identify candidate biomarkers of CHC with potential diagnostic and therapeutic value.CSF levels of amyloid precursor protein and derivatives (solubleAPPα, APPβ), Aβ42, tau, phosphorylated tau, cell adhesion markers L1CAM, and NCAM-1 but not aquaporin 4 or total protein were increased in untreated CHC.Predictive ability for CHC was strongest for sAPPα, followedby normalized sAPP, tau, APP, and L1CAM.
Access full-length article here: https://www.ncbi.nlm.nih.gov/pubmed/28212403

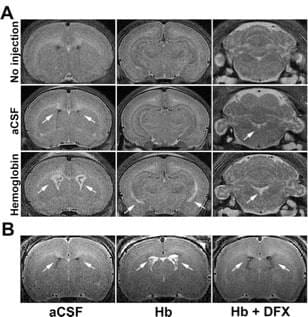
Role of hemoglobin and iron in hydrocephalus after neonatal intraventricular hemorrhage
Neurosurgery. 2014 Dec;75(6):696-705
We reported that intraventricular injections of iron or Hemoglobin(Hb) in P7 neonatal rats resulted in ventriculomegaly, neuronal injury and death. Injection with the iron-deficient immediate heme precursor protoporphyrin IX did not result in ventricular enlargement. Furthermore, treatment with an iron-chelator, deferoxamine, post-Hb injection resulted in significant reduction in Hb-induced hippocampal damage and neuronal injury. These results implicate iron and Hb as key neuropathological substrates of GMH-IVH.This has implications for pathogenesis and treatment of hydrocephalus.
Access full-length article here: https://www.ncbi.nlm.nih.gov/pubmed/25121790

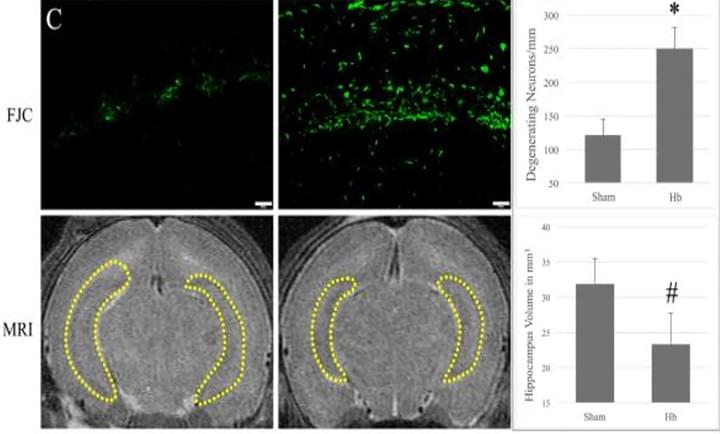
Hemoglobin-induced neuronal degeneration in the hippocampus after neonatal intraventricular hemorrhage
Brain Res. 2016 Mar 15;1635:86-94
We evaluated the role of hemoglobin (Hb) in cell death after intraventricular injection in neonatal rats. Hb was injected into the right lateral ventricle of P7 rats. The CA-1 region of the hippocampus was analyzed via immunohistochemistry, hematoxylin and eosin, Fluoro-Jade C staining, Western blots, and double-labeling stains. Compared to controls, intraventricular injection of Hbdecreased hippocampal volume (27% decrease; p<0.05), induced neuronal loss (31% loss; p<0.01), and increased neuronal degeneration (2.7 fold increase; p<0.01), which were all significantly reduced with the iron chelator, deferoxamine.
Access full-length article here: https://www.ncbi.nlm.nih.gov/pubmed/26772987

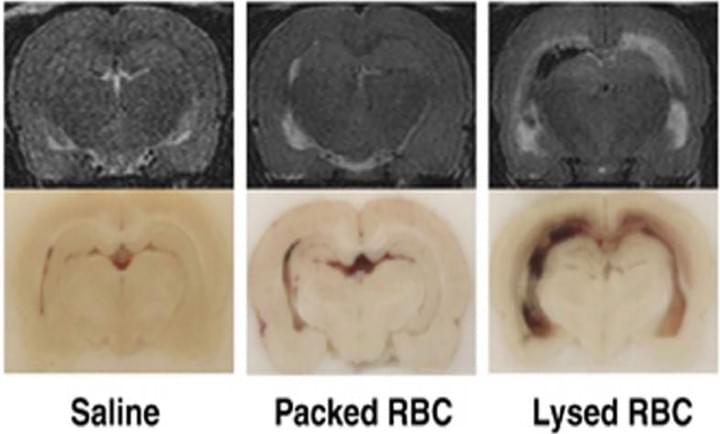
Role of red blood cell lysis and iron in hydrocephalus after intraventricular hemorrhage
J Cereb Blood Flow Metab. 2014 Jun;34(6):1070-5
In this study investigated the role of red blood cell (RBC) lysis and iron in hydrocephalus after IVH. We found that intraventricular injection of lysed RBCs, but not packed RBCs, resulted in ventricular enlargement and marked increases in brain hemeoxygenase-1 and ferritin at 24 hours. Intraventricular injection of iron also resulted in ventricular enlargement and ventricular wall damage 24 hours later. Coinjection of deferoxamine reduced lysed RBC-induced ventricular enlargement. These results suggest that iron, a degradation product of hemoglobin, has an important role in hydrocephalus development after IVH.
Access full-length article here: https://www.ncbi.nlm.nih.gov/pubmed/24667910

Subarachnoid hemorrhage- induced hydrocephalus in rats
Stroke. 2013 Feb;44(2):547-50.
Hydrocephalus is an important complication of subarachnoid hemorrhage (SAH). SAH was induced by endovascular perforation in adult male Sprague-Dawley rats. Ventricular volume measurements showed a significant increase in surviving SAH rats when compared to sham controls. Ventricular volume correlated with SAH severity as demonstrated by histology, immunohistochemistry, Perls’ staining and Western blot analysis. Periventricular iron deposition was observed and HO-1 and Iba-1 expression were markedly increased in hydrocephalus rats.
Access full-length article here: https://www.ncbi.nlm.nih.gov/pubmed/23212164
SELECTED EXPERIMENTAL TECHNIQUES
We use a multifaceted approach to study post-hemorrhagic hydrocephalus spanning both the lab and clinic. We utilize neonatal animal models of post-hemorrhagic hydrocephalus, primary cell culture, human CSF analysis, protein and RNA quantification (ELISA, Western blot, rt-PCR), cilia structure and function assays, CSF outflow quantification and behavior studies.

T2-Weighted MRI
To evaluate ventricle size and brain injury, we use in vivo MRI to quantify ventricular volumetric measurements and assess brain injury.
Cilia Beat Frequency Measurements
As cilia function is implicated in hydrocephalus, we evaluate cilia structure and function in cell culture and after intraventricular hemorrhage in our neonatal rodent model.

X-ray nanotomography
In collaboration with the WUCCI, we evaluate CSF pathways through the brain and spinal cord parenchyma using gold nanoparticle-enhanced x-ray nanotomography to obtain high-resolution images of CSF circulation through the brain, neck, and spine.

tHE PI - Dr. Jennifer M. Strahle
Dr. Strahle received her undergraduate degree from Bates College where she double majored in neuroscience and biochemistry. She received her MD from the University of Minnesota and completed her neurosurgical residency at the University of Michigan. She came to Washington University in Saint Louis for pediatric neurosurgery fellowship training in 2015. Dr. Strahle is currently an Associate Professor of Neurological Surgery, Orthopedic Surgery and Pediatrics and the Director of Pediatric Neuro Spine Program. In addition to hydrocephalus, her clinical interests are in pediatric vascular disease including moyamoya, pediatric spine tumors, spinal deformity, brain tumors, craniocervical junction disorders, spina bifida, Chiari malformations, neuro-endoscopy, pediatric spine trauma, syringomyelia, and CSF circulation. She conducts basic science and clinical research focused on advancing the field of pediatric neurosurgery through a greater understanding of disease processes, such as post-hemorrhagic hydrocephalus and advancement of treatment strategies.
Meet OUR TEAM
We are a multitalented group of committed scientists and medical students driven to making fundamental discoveries in the lab and rapidly translating these insights to the clinic.

Lihua Yang, MD, PhD
Senior Scientist
Lihua is a senior scientist in the Strahle and Rubin labs. She receives her MD from China and PhD from Japan. She has worked in internal medicine department in China. After PhD graduation, she has worked as an assistant professor in Ehime University in Japan. She started working in Wash.U. in 2004. She work in Rubin lab as a senior scientist, research on brain tumors to improve clinic efficiency. Her projects focus on the genesis of brain tumors, intracranial growth of medulloblastoma and glioblastoma, and the development of novel therapeutics. In her free time, she enjoys Chinese painting, walking with her dog, taking care of plants.

Diego Morales, MS
Staff Scientist

Shelei Pan
Medical Student
Shelei is an incoming M1 at Washington University in St. Louis School of Medicine. She graduated from Washington University in December 2023 with a major in Biology (neuroscience track) and minor in Spanish. She joined the Strahle lab as a high schooler in December 2019. Shelei studies targeted CSF interactions with the parenchyma, CSF pathways in post-hemorrhagic hydrocephalus, and the molecular basis of CSF handling in the developing brain.

Grace Halupnik
Research Technician
Grace Halupnik graduated from Washington University with a major in Psychology-Neuroscience-Philosophy program and a minor in biomedical physics. Grace joined the lab in 2022 and studies CSF pathways in post-hemorrhagic hydrocephalus as well as CSF circulation during lymphatic and vascular development.

Shriya Koneru
Undergraduate Student
Shriya is an undergraduate at Washington University in St. Louis studying Biology with a concentration in Neuroscience and minoring in Anthropology. Shriya joined the Strahle Lab in 2022, where she has been studying CSF-cerebellar interactions in the context of IVH and resting state functional network alterations after GMH-IVH.

Ashlynn Lizer
Research Technician
Ashlynn Lizer did her undergraduate at Drake University where she majored in Health Sciences: Clinical and Applied with a minor in psychology. Ashlynn joined the lab in the summer of 2024. She is doing a mix of basic lab research and clinical research.

Peter Yang, MD
Neurosurgery Resident
Peter joined the Strahle lab in Summer 2021 as a PGY6 neurosurgery resident. Peter studied CSF interactions with the parenchyma in development, transependymal CSF migration, and functional network alterations following GMH/IVH.

Abdul-Haq Alli
Undergraduate Student
Abdul-Haq is an undergraduate at Washington University in the class of 2024. He joined the Strahle lab in 2021. Abdul-Haq studies rostral migratory stream development following IVH-PHH.

Natalia Alonzo
Rotation Student
Natalia is a first year PhD student currently rotating in the Strahle Lab. She went to Boston University and received her Bachelor’s degree in Biomedical Engineering as well as a minor in Innovation & Entrepreneurship before coming to WashU. Natalia first developed an interest in research after completing summer internships in various R&D departments of Corning Incorporated as a high school student. Through both personal and professional experiences in her undergraduate career, she developed a passion for women’s health research in particular. In the Strahle Lab, she is interested in using different imaging modalities to track CSF flow and develop an understanding of the glymphatic system’s role in CSF transport.
Sangami Pugazenthi
Medical Student
Resident Trainee Specialty Decision Making and Perceptions of Neurosurgery; Departmental Grant for survey work on medical student decision making.
Sneha Chaturvedi
Medical Student
Circadian Rhythms impact on CSF pressure and production in pediatric ICU patients.
Ashely Dunbar, MD
Neurosurgery Resident
Brain Development in myelomeningocele: Prenatal vs. Postnatal Closure
Matthew ReVeal
Medical Student
Medical Student, Natural History of Klippel Fiel Anomaly
Diane Aum, MD
Neurosurgery Resident
Supratentorial Network Alterations by Posterior Fossa Tumors, Neurosurgery Research and Education Foundation Research Fellowship Grant
Sasidhar Karuparti
Medical Student
University of Missouri Medical School, Cranial growth trajectories and ventricular enlargement in prenatally vs. postnatally treated myelomeningocele. 2022 Clinical Research Training Center’s Summer Predoctoral Program
Joshua Koleske
Resident
Leptomeningeal and dural CSF handling; Circadian variations in CSF solutes.
Former Members
Prabagaran Esakky, PhD
Senior Scientist
Praba got his PhD in Biochemistry with specialization in Male Reproductive Toxicology from (ICMR) University of Mumbai in 2008. He completed his postdoctoral trainings in Developmental Neurobiology at WEHI in Melbourne, Australia (2008-2009), Vascular Physiology at UTHSC, Memphis (2009-2010), followed by training in molecular reproductive toxicology in the Department of OB & GYN at Washington University in St. Louis (2010-2015). Following postdoc trainings, Praba has made significant scientific contributions as a Research Instructor in the department until 2019. His research has primarily focused on unraveling the harmful effects upon paternal exposure to environmental pollutants and germ cell toxicants such as constituents of cigarette smoke and viral pathogens like ZIKA virus on male fertility, male germ cell development, birth defects in embryos and offspring, and developmental and neurobehavioral outcomes in offspring. Praba has joined the Strahle Lab in June 2019 and is currently focusing on the role of proteins in ventricular injury and ependymal ciliary dysfunction and its impact on CSF dynamics in GMH-IVH.
Gretchen Koller
Medical Student
Gretchen Koller is a medical student from Kansas City University completing a predoctoral research fellowship with the Strahle Lab and WashU Department of Neurosurgery. She is also earning a Master of Public Health from the University of Nebraska Medical Center. She obtained her Bachelor of Science in biological sciences from the University of Missouri – Columbia, where she conducted research on proinflammatory mediators and their effect on capillary tube regression. While in medical school, she has devoted her time to working with the Medical Student Neurosurgery Training Center and leads their affiliated Neurosurgery Education and Research Virtual Group. Her research interests include congenital malformations within and related to the central nervous system, pediatric neuro-oncology, and clinical outcomes of fetal surgery. In addition to her academic and research pursuits, Gretchen is passionate about increasing involvement and representation of women within the field of neurosurgery and providing equal access to neurosurgical education. She is originally from Ste. Genevieve, Missouri, and enjoys the beautiful wine country, creating charcuterie boards, playing volleyball and racquetball, hosting friends and family, baking, biking, and hiking.
Bing Xue, MD
Staff Scientist
Dr. Xue received his M.D. degree from Taishan Medical College in China in July 2002. He received his Ph.D. in Anatomy and Embryology from Ehime University School of Medicine in Japan in July 2007. Dr. Xue completed his Post-doctoral training in Physiology at the University of Kansas Medical Center from 2007 to 2008, followed by training at the University of Missouri-Kansas City School of Medicine from 2008 to 2016. He joined the Strahle Lab in March 2017. Dr. Xue focused on the role of iron transporters and cilia dysfunction and effects on regional and global CSF flow after GMH-IVH.
Sharon Abada, MD
Medical Student
Prior to attending medical school at Washington University in St. Louis, Sharon Abada received her Bachelor's degree in Physiology from UCLA and her Master's in Public Health from the UTHealth School of Public Health. She joined the Strahle Lab in the summer of 2018. In the lab, she worked on studying the role of ependymal iron transporters in post-hemorrhagic hydrocephalus using a neonatal rodent model.
Chandana Buddhala, PhD
Senior Scientist
Dr. Buddhala received her Ph.D. in Integrative Biology from Florida Atlantic University in May 2012. She completed Post-doctoral training with Dr Paul Kotzbauer in the Department of Neurology at Washington University in Saint Louis in May 2017. She joined the Strahle Lab in July 2017. Dr. Buddhala contributed to findings on Intraventricular Hemorrhage Clearance Markers in Human Neonatal CSF. She focused on CSF iron metabolism predictors of outcome in post hemorrhagic hydrocephalus, iron-mediated ventricular injury and cilia dysfunction in post hemorrhagic hydrocephalus.
Mounica Paturu, MD
Medical Student
Mounica Paturu received her Bachelor of Science from Massachusetts Institute of Technology where she double majored in Bioengineering and International Development. She joined the the Strahle Lab in 2018. Ms. Paturu was involved in clinical research evaluating the relationship of ventricular size to CSF biomarkers of post-hemorrhagic hydrocephalus.
Alex Skidmore, MD
Medical Student
Alex Skidmore completed his Bachelor of Science in Neuroscience from Duke University. He joined the Strahle Lab in the summer of 2018. Alex focused on studying changes in iron transporter expression in hemoglobin and iron-mediated injury in neonatal rat primary cultured ependymal cells.
Jordan Gewirtz
Undergraduate Student
Jordan Gewirtz is a graduate of Washington University in St. Louis and studied systems engineering and computer science on a pre-medicine track. He joined the Strahle Lab in the summer of 2019. In the lab, he worked on the Park-Reeves Research Consortium focusing on Chiari, syringomyelia, and scoliosis as well as validating a modified spine MRI protocol.
Dhvanii Raval
Undergraduate Student
Dhvanii Raval is a 2022 graduate of Saint Louis University. She joined Strahle Lab in February 2020. In the lab, she focused on determining CD163 expression in a rat IVH-PHH model.
Dakota DeFreitas, MA
Research Technician
Dakota received his Masters in Neurobiology at Washington University in St. Louis. He focused on the role of iron transporters in iron-mediated ventricular injury and behavioral deficits after post-hemorrhagic hydrocephalus.
Jordann Wilson, MS
Medical Student
Jordann joined the lab as a 2022 Black Lives Matter summer scholar and is a medical student in the Drexel University College of Medicine. She is passionate about addressing health disparities in all forms of medicine and looks to serve marginalized and impoverished areas in the future.
Emily Johnson, MD
Medical Student
Cardiac contributions to intracranial CSF, Helmeting for plagiocephaly in shunted hydrocephalus.
Sruthi Ramagiri, PhD
Staff Scientist
Sruthi Ramagiri received her Ph.D. in Neuroscience from BITS Pilani in May 2018. She completed her Post-doctoral training in the Department of Pediatrics at Albert Einstein College of Medicine in New York in June 2019. She joined the Dr. Strahle lab in July 2019. Dr. Ramagiri is currently focusing on the role of iron transport at the ependymal surface and its contribution to ciliary dysfunction in IVH-PHH.
John O'Malley
Medical Student
University of Missouri Medical School, Choroid Plexus Development in Preterm Brain Injury, 2021 Clinical Research Training Center’s Summer Predoctoral Program
Mackenzie Lemieux
Medical Student
University of Missouri Medical School, Choroid Plexus Development in Preterm Brain Injury, 2021 Clinical Research Training Center’s Summer Predoctoral Program
Hello & Welcome!
Thank you for your interest in joining the Strahle Lab. If you are a bright, enthusiastic and hard working individual who is excited to learn and contribute to hydrocephalus research, you are at the right place.
Research Staff Scientists, Post-Doc Research Associates and Technicians:
We are actively recruiting highly motivated Post-Doctoral Research Associates, Staff Scientists and Technicians. Please send a cover letter and CV to Dr. Strahle at strahlej@wustl.edu.
Medical Students:
Medical students typically enter the lab as a summer research student between the first and second year of medical school. They may continue to be involved in laboratory or clinical research and/or set aside a dedicated research year in the lab.
Undergraduate Students:
We request that interested students are willing to commit at least one summer (full time) or approximately 10 hours per week. If interested please send a CV/resume to Dr. Strahle at strahlej@wustl.edu.
NEWS
Disrupted flow of brain fluid may underlie neurodevelopmental disorders
New imaging technique reveals circulation patterns in developing brain
Click here for more info:
https://medicine.wustl.edu/news/disrupted-flow-of-brain-fluid-may-underlie-neurodevelopmental-disorders/
2016 Neurosurgeon Research Career Development Program Award Recipient
Click here for more info:
2016 Hydrocephalus Association Innovator Award Recipient
This award was made possible through the generous support of Team Hydro.
Click here for more info:
https://www.hydroassoc.org/innovator-award-investigators/jennifer-strahle-md/
- specIal thanks
We couldn't have done it alone without continued support from our patients, families and funding sources.

- D O N A T E
All our research is conducted with the patient in mind. We continuously initiate new research projects across multiple areas within the realm of hydrocephalus to find a cure. If you would like to support this important effort through your donation, please feel free to contact:
Washington University School of Medicine
Pam Morris
314-935-9693
morrisp@wust.edu

Jennifer M. Strahle
Associate Professor
Department of Neurosurgery
Washington University School of Medicine
One Children's Place
Suite 4s20
Saint Louis, MO 63110
ph: 314-454-2816
strahlej@